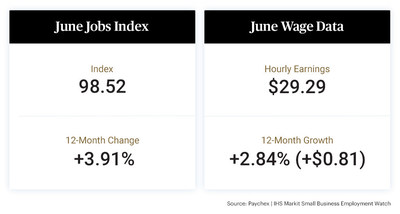
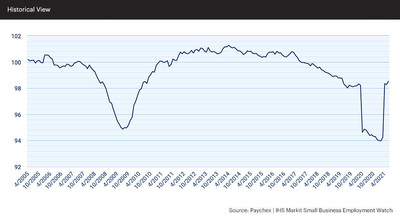
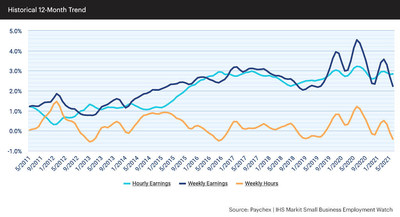
SAN DIEGO, June 29, 2021 (GLOBE NEWSWIRE) — Genasys Inc. (NASDAQ: GNSS), the global leader in critical communications systems and solutions, today announced international LRAD 1000Xi systems orders totaling $1.0 million for critical infrastructure protection and wildlife preservation.
Offshore oil platforms in Nigeria will be equipped with LRAD 1000Xi systems to communicate to and warn away security threats, encroaching fishing boats, and approaching vessels not responding to radio calls.
“Flags, lights and flares are ineffective in stopping encroaching boats from entering restricted areas around oil & gas platforms,” said Richard S. Danforth, Chief Executive Officer of Genasys Inc. “LRAD’s long-range alert tone and exceptionally clear voice broadcasts provide platform security personnel more time to determine the intent of approaching vessels and scale responses appropriate to the incursions.”
The orders also include LRAD systems integrated with avian radar from DeTect, Inc. to humanely deter waterfowl and other wildlife from entering hazardous water and waste areas at a large mining operation in northern Canada.
“LRAD systems use directionality and focused acoustic output to broadcast a near infinite variety of predator calls and warning tones to ensure against wildlife habituation,” added Mr. Danforth. “In addition to significantly reducing waterfowl deaths at mining operations, LRADs are in use throughout the world to preserve wildlife and protect critical assets at airports, air bases, oil & gas facilities, and fisheries.”
LRAD systems broadcast audible voice messages and tones with exceptional clarity from close range to 5,000 meters. Rugged, reliable, and built to withstand harsh environments, LRAD systems help resolve uncertain situations and save lives.
About Genasys Inc.
Genasys™ is a global provider of critical communications solutions that help keep people safe. Genasys systems are in service in more than 100 countries around the world in a range of diverse applications, including defense, public safety, national emergency warning systems, mass notification, law enforcement, critical infrastructure protection, and many more. For more information, visit genasys.com.
Forward-Looking Statements
Except for historical information contained herein, the matters discussed are forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934. You should not place undue reliance on these statements. We base these statements on particular assumptions that we have made in light of our industry experience, the stage of product and market development as well as our perception of historical trends, current market conditions, current economic data, expected future developments and other factors that we believe are appropriate under the circumstances. These statements involve risks and uncertainties that could cause actual results to differ materially from those suggested in the forward-looking statements. These forward-looking statements are subject to a number of risks and uncertainties, including without limitation our ability to recognize the expected synergies and other benefits of the Zonehaven acquisition; difficulties in integrating Zonehaven post-closing; diversion of management time addressing post-closing transaction-related issues; uncertainties related to litigation involving the acquisition of Zonehaven; uncertainties related to unanticipated integration costs or undisclosed liabilities assumed; uncertainties related to the acceptance of the Zonehaven acquisition and its products by third parties; the business impact of health crises or outbreaks of disease, such as epidemics or pandemics and how they may affect our supply chain, and other risks and uncertainties, many of which involve factors or circumstances that are beyond the Company’s control. Risks and uncertainties are identified and discussed in our filings with the Securities and Exchange Commission. These forward-looking statements are based on information and management’s expectations as of the date hereof. Future results may differ materially from our current expectations. For more information regarding other potential risks and uncertainties, see the “Risk Factors” section of the Company’s Form 10-K for the fiscal year ended September 30, 2020. Genasys Inc. disclaims any intent or obligation to update those forward-looking statements, except as otherwise specifically stated.
Investor Relations Contacts Jim Fanucchi and Satya Chillara Darrow Associates, Inc. [email protected]